
GLASSIA® offers the most
infusion setting options for
patients with emphysema caused by
severe Alpha-1 antitrypsin deficiency
No two alphas
are alike.

self-infusion

with nurse

center

Glassia is the Alpha-1 augmentation therapy with the most infusion setting options, so you and your doctor can choose what works best for you.
†If self-infusion is deemed appropriate, ensure that you
receive detailed instructions and adequate training on how to
infuse at home or other appropriate setting and have
demonstrated the ability to independently administer GLASSIA.
LINDA
Real GLASSIA patient since 2011
Self-Infuses at home
What is GLASSIA?
GLASSIA is a medication used to treat adults with lung disease (emphysema) caused by severe Alpha-1 antitrypsin deficiency (AATD). People living with AATD produce little to no Alpha-1 antitrypsin protein, so GLASSIA works to increase Alpha-1 protein levels in the blood and lungs, while also giving patients options on the setting in which they receive their infusions.

Freedom for Linda
Linda loves to travel and explore new places, and self-infusing gives her the freedom to administer GLASSIA once a week on her schedule—even when she travels.‡
‡Store GLASSIA at 2 °C to 8 °C (36 °F to 46 °F). Do not freeze. Product may be stored at room temperatures not exceeding 25 °C (77 °F) for up to 1 month. Once removed from refrigeration, use within 1 month.
Go to Linda's Story
Convenience for Shannon
Shannon likes having a nurse to administer GLASSIA at his house so he can receive his weekly infusion and get on with his day.
Go to Shannon's Story
Flexibility for Vanessa
Vanessa prefers to receive GLASSIA at the infusion center so she can keep up with her weekly treatment and her daughters’ schedules.
Go to Vanessa's Story
Is self-infusion at home
right for me?
Some Alphas learn how to self-infuse with GLASSIA after training with a nurse. See what it’s like to self-infuse with a caregiver’s help.¶
¶If self-infusion is deemed appropriate, ensure that you receive detailed instructions and adequate training on how to infuse at home or other appropriate setting and have demonstrated the ability to independently administer GLASSIA.
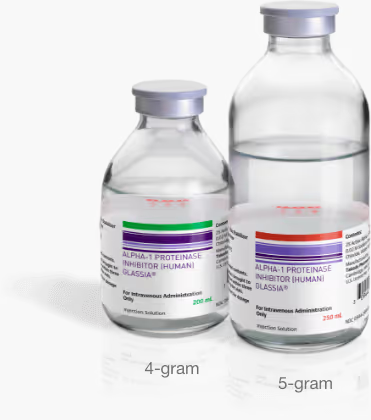
GLASSIA’s larger vial options could help streamline your infusion
Larger vials mean fewer vials§ need to be used in the infusion process. In addition to 1-gram vials, GLASSIA offers 4- and 5-gram vial sizes that could help simplify your infusion process with fewer supplies. Learn how larger sizes could mean fewer vials for each infusion.
Explore vial size options§For Alpha-1 patients who previously needed four or more 1-gram vials per infusion, due to GLASSIA’s weight-based dosing requirements.

What to know about Alpha-1 antitrypsin deficiency (AATD)
Find out more about who may have Alpha-1 deficiency, what symptoms look like, and how to talk to your doctor about managing it.

Where can I find out about how to manage my severe Alpha-1 deficiency with GLASSIA?
Download information about what to expect when you choose GLASSIA infusion treatment for your Alpha-1 antitrypsin deficiency.

Welcome to Takeda Patient Support
—support specialists you can
count on
Takeda Patient Support is a product support program for people who have been prescribed GLASSIA. Our support specialists are here to address your questions and concerns and help get you the answers, resources, and tools you need. Some of the ways we can assist include:
- Enrolling
you in the Takeda Patient Support Co-Pay Assistance Program, if you qualify# - Working
with your specialty pharmacy (or site of care) to help you receive GLASSIA - Arranging
for a trained nursing professional to teach you or a caregiver how to infuse your treatment at home, if requested by your healthcare provider - Navigating
the health insurance process - Directing
you to community support resources and education - Providing
you with tips and timely information throughout your GLASSIA treatment
#To be eligible, you must be enrolled in Takeda Patient Support and have commercial insurance. Other terms and conditions apply. Call us for more details.
Not enrolled and want to get started? Here’s what to do:
- Work with your healthcare provider to complete the Start Form for your prescribed Alpha-1 therapy
- Sign the Patient Authorization section of the Start Form
- Takeda Patient Support will confirm your eligibility
- Your dedicated support specialist will contact you
Have questions? We’re here to help.
Please visit
TakedaPatientSupport.com/Glassia

Stay connected. Stay informed.
Sign up for more information about GLASSIA and AATD treatment.
Sign up for updates
