
LINDA
Real GLASSIA patient since 2011
Self-infuses at home
WITH GLASSIA® AUGMENTATION THERAPY,
YOU CAN GIVE YOUR PATIENTS THE
freedom to infuse
on their terms

self-admin

with nurse

center

You can offer your Alpha-1 patients‡ a weekly infusion with the most options for administration settings, including self-administration at home. Together, you can decide what works best for them.
†If self-administration is deemed appropriate, ensure that the patient/caregiver receives detailed instructions and adequate training on how to administer in the home or other appropriate setting and has demonstrated the ability to independently administer GLASSIA.
‡Alpha-1 patients are defined as patients who have Alpha-1 antitrypsin deficiency (AATD).
Get your patients started on GLASSIA.
From information about Alpha-1 antitrypsin deficiency (AATD) to GLASSIA coverage for your patients, you will find what you need here.

Initiate GLASSIA treatment for your Alpha-1 patients.
Initiate treatment using the Start Form and follow the steps for getting your patient started with GLASSIA.

Resources to help you get your patients started on GLASSIA
Find a variety of resources, including our Dosage and Administration Guide and forms for your practice.
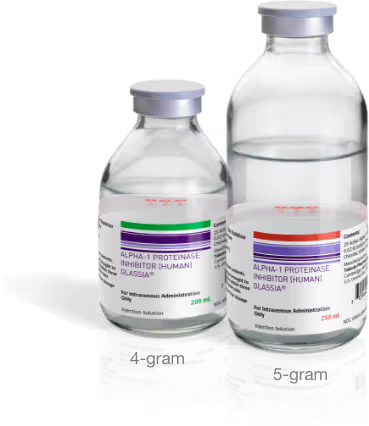
Help simplify infusions for your patients with larger vial options.
GLASSIA offers multiple vial size options for your patients. Larger 4- or 5-gram vials could even offer a streamlined infusion experience with fewer supplies and fewer vials for each infusion.¶
EXPLORE VIAL SIZE OPTIONS¶For Alpha-1 patients who previously needed four or more 1-gram vials per infusion, due to GLASSIA’s weight-based dosing requirements.

Welcome to
Takeda Patient Support
—support specialists your patient can count on
When you prescribe GLASSIA for your patient, Takeda Patient Support is here for them. Our support specialists can address your patient’s questions and concerns and help get them the information they need.
For onboarding, access, and reimbursement assistance, our services include:
- Benefits investigation to help determine your patient’s insurance benefits and eligibility for certain services
- Prior authorization (PA), reauthorization, and appeals information
- Information about financial assistance options for your patient, if they're eligible
Our additional services include:
- Specialty pharmacy (or site of care) triage and coordination
- Takeda product infusion training for healthcare providers, as well as home treatment support and education for your patient
- Arranging for a trained nursing professional to teach your patient or their caregiver how to infuse treatment at home, if requested by your office
- Directing your patient to community support resources
What to know about Alpha-1 antitrypsin deficiency (AATD) and how to treat it with GLASSIA
GLASSIA is a medication clinically proven to help increase Alpha-1 levels.1
GLASSIA (Alpha1-Proteinase Inhibitor) is a liquid augmentation therapy prescribed by pulmonologists for over a decade to help increase Alpha-1 levels.
Underdiagnosis of Alpha-1 antitrypsin deficiency is common.2 Here’s why.
AATD can be difficult to diagnose because it causes lung disease that mimics other lung conditions. When should Alpha-1 testing be performed?
Stay connected. Stay informed.
Sign up for more information about GLASSIA.
References
- GLASSIA. Prescribing information. Takeda Pharmaceuticals U.S.A., Inc.; 2025.
- Campos MA, et al. Chest. 2005;128(3):1179-1186.

![GLASSIA [Alpha1-Proteinase Inhibitor (Human)] logo.](/dist/images/hcp/glassia_logo.svg)