
SHANNON
Real GLASSIA patient since 2022
Infuses at home with a nurse
GLASSIA Dosing and Administration for Alpha-1 Patients
WITH FLEXIBILITY TO CHOOSE AN INFUSION SETTING

self-admin

with nurse

center

The only FDA-approved, self-administration* at home option for patients to help raise their Alpha-1 levels1
*If self-administration is deemed appropriate, ensure that the patient/caregiver receives detailed instructions and adequate training on how to administer in the home or other appropriate setting and has demonstrated the ability to independently administer GLASSIA.
GLASSIA is the first FDA-approved† liquid treatment for Alpha-1 antitrypsin deficiency (AATD) and has a weekly dosage.1
GLASSIA’s infusion rate is ~15 minutes.
- GLASSIA’s infusion time is based on a maximum rate of 0.2 mL/kg/min and a recommended dosage of 60 mg/kg1
GLASSIA is an AATD treatment that comes as a ready-to-administer liquid, so your patients and their caregivers will not need to mix anything before their infusion.
- With no reconstitution required, GLASSIA’s ready-to-administer formulation helps decrease administration steps—and the potential for preparation errors1,2
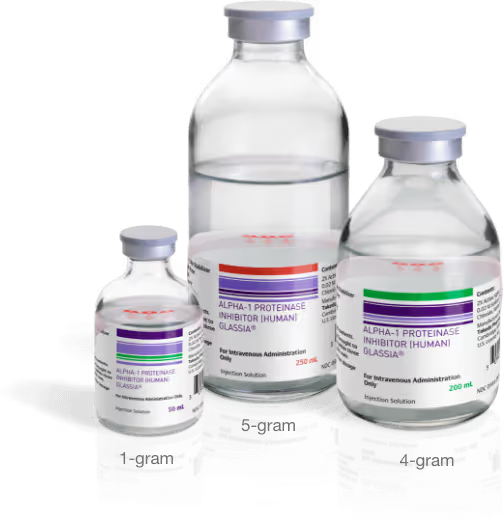
- Each single-use vial of GLASSIA contains at least 1 gram (1000 mg) of functional Alpha-1 PI in 50 mL of solution. Vial size options include 1, 4, or 5 grams of functional Alpha-1
†GLASSIA was approved by the FDA on July 1, 2010.
Download the GLASSIA Dosage and Administration Guide for dosing and ordering information and step-by-step details on administration for in-office or at-home infusions.
Download the glassiadosage & Administration Guide

When you and your patient have chosen a treatment path
When your patient is prescribed a Takeda treatment, Takeda Patient Support is dedicated to helping them get the answers, resources, and tools they need.
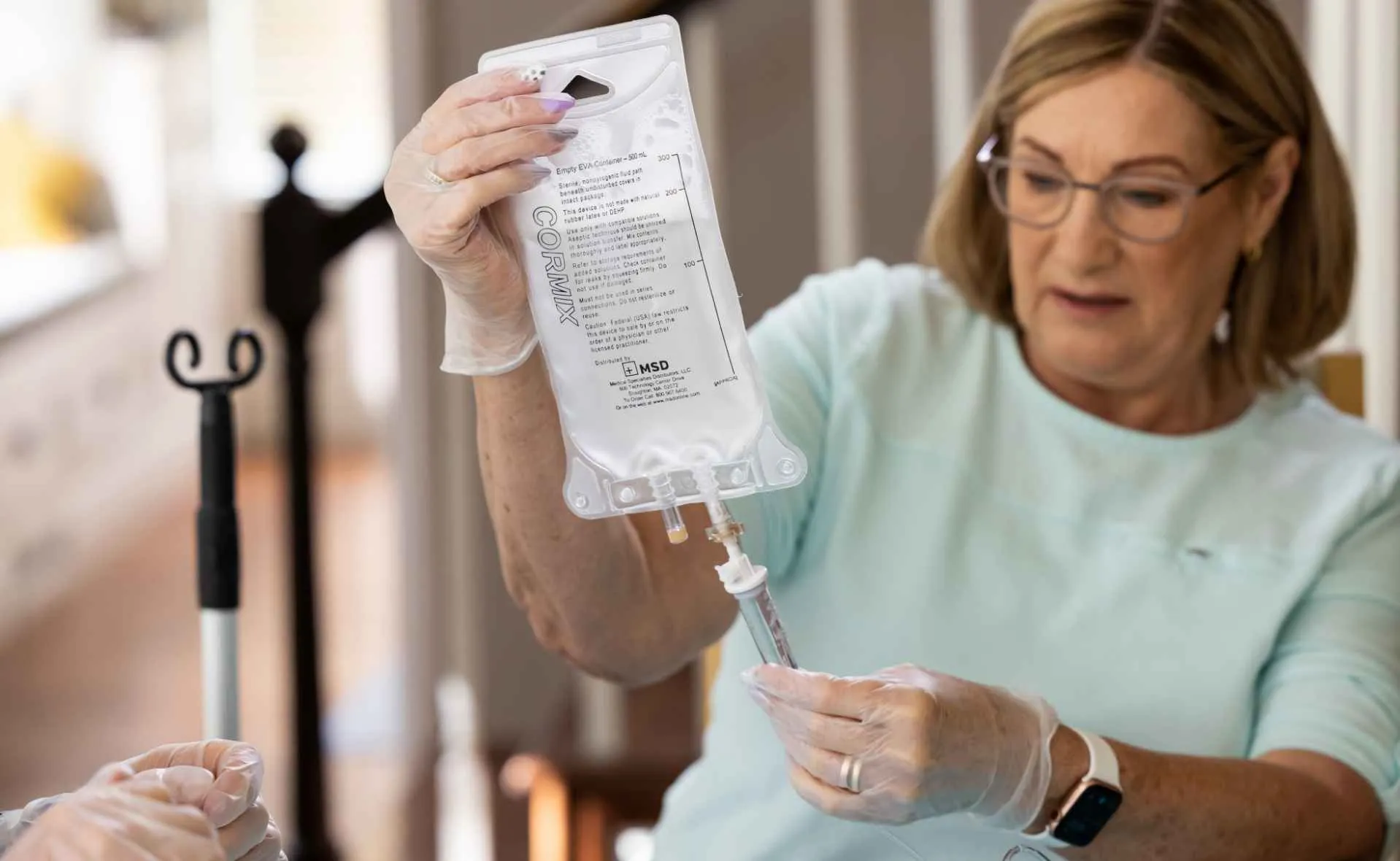
GLASSIA is the first and only AATD augmentation therapy approved for self-administration after appropriate training.
Keep in mind that patients must be trained before getting started—but we offer resources to support that process.
While self-infusion may seem complex at first, we’re here to help make it approachable. Our Welcome Kit includes printed materials and a step-by-step instructional video that walks patients through the entire process—from infusion prep to administering directly from the vial using the vial hanger label. And once you prescribe GLASSIA, our support programs are ready to help your patients get started.
Find ResourcesGLASSIA vial size options can offer more convenience.‡
With larger vial sizes, the infusion process could be more convenient for you and your patients. GLASSIA’s 4- and 5-gram vials could better accommodate those who require a larger dose, while also offering other benefits, including:

Less pooling
Whether they infuse with a healthcare professional or self-administer at home, less pooling could be required.
Fewer vented spikes and transfer needles
Because fewer vials are required for an infusion, fewer vented spikes and transfer needles may be used.¶

Saving space
Less shelf space may be required in infusion centers, doctors’ offices, at specialty pharmacies, and homes for patients who self-administer.§
Changing to larger vials shouldn’t affect a patient’s insurance coverage or how they receive their product, and the ordering process should stay the same.
‡Depending on prescribed dose.
¶For Alpha-1 patients who previously needed four or more 1-gram vials per infusion, due to GLASSIA’s weight-based dosing requirements.
§4 g and 5 g vials occupy 50% and 40% less space than their 1 g equivalents, occupying 58 cm2 versus 116 cm2 and 146 cm2 for 4 and 5 of the 1 g vials.
Important considerations while infusing
GLASSIA is administered weekly with a dosage of 60 mg/kg body weight. Things to keep in mind for a smooth infusion process include:
- Closely monitoring the infusion rate to catch infusion-related reactions
- Storing GLASSIA in an appropriate location
- Making sure the product reaches room temperature prior to infusing
For more info, take a look at our Dosage and Administration Guide.
GLASSIA has a demonstrated safety profile.
Learn more about the most common adverse effects and reactions.
Stay connected. Stay informed.
Sign up for more information about GLASSIA.
References
- GLASSIA. Prescribing information. Takeda Pharmaceuticals U.S.A., Inc.; 2025.
- European Medicines Agency. Good practice guide on risk minimisation and prevention of medical errors.
https://www.ema.europa.eu/documents/regulatory-procedural-guideline/good-practice-guide-risk-minimisation-prevention-medication-errors_en.pdf. Accessed September 14, 2023.
![GLASSIA [Alpha1-Proteinase Inhibitor (Human)] logo.](/dist/images/hcp/glassia_logo.svg)